Project Description
PMTC

Project Description
The Pharmaceutical Manufacturing Technology Centre (PMTC) has a clear vision to make Ireland the global hub of pharmaceutical process innovation and advanced manufacturing through applied research into advanced technology solutions. The PMTC was established in December 2013 and is led by an industry steering board with an active research programme in leading Irish RPOs, driven by its industry members. Companies access PMTC to create projects & execute world-beating industry-relevant research in advanced technology solutions which address contemporary manufacturing issues. PMTC focuses on a number of different research themes, addressing issues such as Continuous Processing, Plant Cleaning Processes & Verification, Powder Processing and Chemometrics.
CAPPA Involvement
CAPPA has been involved in a number of projects within the PMTC, and is currently a partner in a PMTC Core-Funded research project on cleaning validation. Cleaning validation is a required activity within the pharmaceutical and biological industries. It has a massive impact on plant efficiency, utilities and resources. In some cases as much as 50% of time is spent engaged in cleaning, resulting in a massive impact on downtimes, costs and changeovers. This has become even more problematic in recent years with an increasing focus on smaller production volumes resulting in increased numbers of changeovers and more time spent devoted to cleaning. From both a regulatory and industry standpoint, cleaning validation is recognised as an important activity to establish that product cross contamination is controlled to ensure patient safety and product quality, and forms an important component of Process Analytical Technology (PAT). Cleaning validation is an on-going activity within these cGMP compliant environments, which necessitates the investment of significant resources and time. Current analytical ways for validation of the cleanliness of the environment include; HPLC/UPLC, Total Organic Carbon (TOC), UV and conductivity.
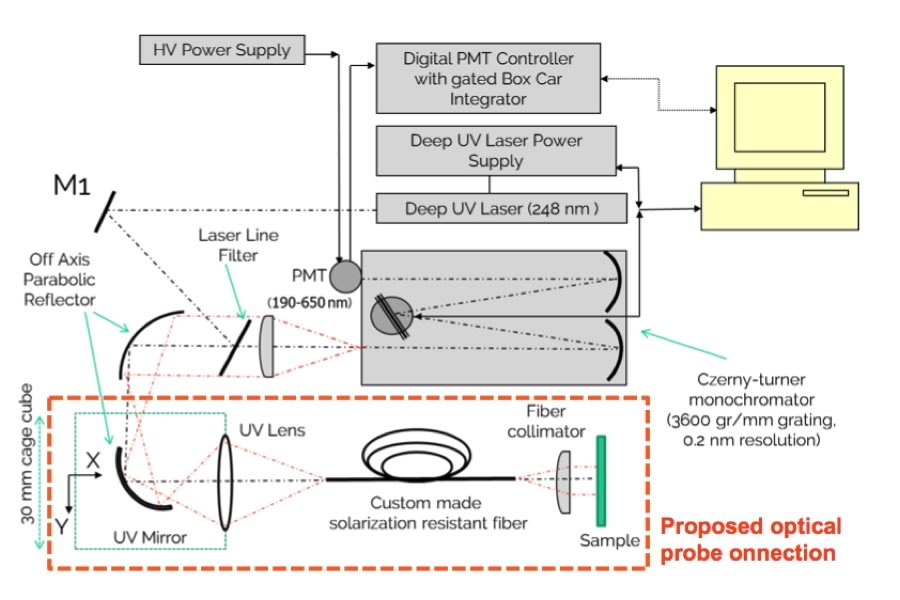
Deep UV Resonance Raman Spectroscopy for Cleaning Verification
The objectives of this project are to build a bench top prototype to determine the detection/sensitivity of Deep UV Resonance Raman Spectroscopy for use with the pharmaceutical excipients, APIs and cleaning agents, and to investigate the applicability of using an optical fibre to develop a contact probe for hard to reach areas. Deep UV Resonance Raman Spectroscopy (DUVRRS) provides a combined Raman and fluorescence spectra measurement with improved detection limits versus Surface-Enhanced Raman spectroscopy (SERS), and a considerable feedback on contaminant levels and identity can be achieved with minimal sample preparation. Specific improvements on existing technologies like HPLC are contained within an easy-to-use analysis protocol.
Industry Impact
Rapid analysis and feedback on contaminant levels and identity can be achieved with minimal sample preparation. Specific improvements on existing technologies like HPLC are contained within an easy to use analysis protocol. Specifically:
- Significantly improved sample response times which prevents a measurement backlog
- Identification of molecular functional groups allows end users to apply targeted cleaning procedures
- Reduced production downtime from days to hours
- Simultaneous multi-component detection
- Help to identify difficult to clean areas
To find out more about Deep UV Resonance Raman Spectroscopy (DUVRRS) you can download our flyer here.








