Process Monitoring
Process monitoring and control are vital for various industry sectors, pharmaceutical, biopharmaceutical, polymer manufacturing and food and dairy. Typical process monitoring involves the use of temperature, humidity, flow and pressure meters. While these can be robust measures of a process to enable control, it does not always provide sufficient information at critical process parameters (CPP). An all-encompassing term for process monitoring, control and analysis systems is Process Analytical Technology (PAT). These systems can be used to measure critical quality and performance attributes of raw and processed materials, which can lead to improved product quality, reduced time to market, and tighter and more responsive supply chains.
PAT systems can be implemented on-line, in-line, at-line or by-pass and each method come with their own challenge for implementation and inherent befits. CAPPA has been developing PAT systems to meet the needs various industrial needs across a range of sectors and as such has developed bespoke expertise for developing a whole new class of sensing equipment. Some potential applications are outlined below; these are based on spectroscopic techniques – the backbone of CAPPAs knowledge – UV/vis, fluorescence, Raman and Infrared (IR), both near and mid.
Monitoring of moisture level
Infrared spectroscopy is especially suited for sensing of moisture content in a range of processes. The measurement principle is based on IR light absorption by water molecules and applies to both mid-infrared (MIR) and near infrared (NIR). A typical process monitoring application of in-line moisture monitoring would be the freeze-drying of pharmaceutical proteins. Freeze drying includes three different stages, beginning with the freezing procedure followed by the primary drying and finally the secondary drying step – all of which have CPP that require monitoring. A NIR spectroscopic PAT system allows for a non-invasive, non-destructive and rapid moisture determination. The requirement for monitoring moisture levels is not only critical to the pharmaceutical sector; it can also find applications in the food and dairy sector. Raw and final products required monitoring during and after production to avoid microbial growth.
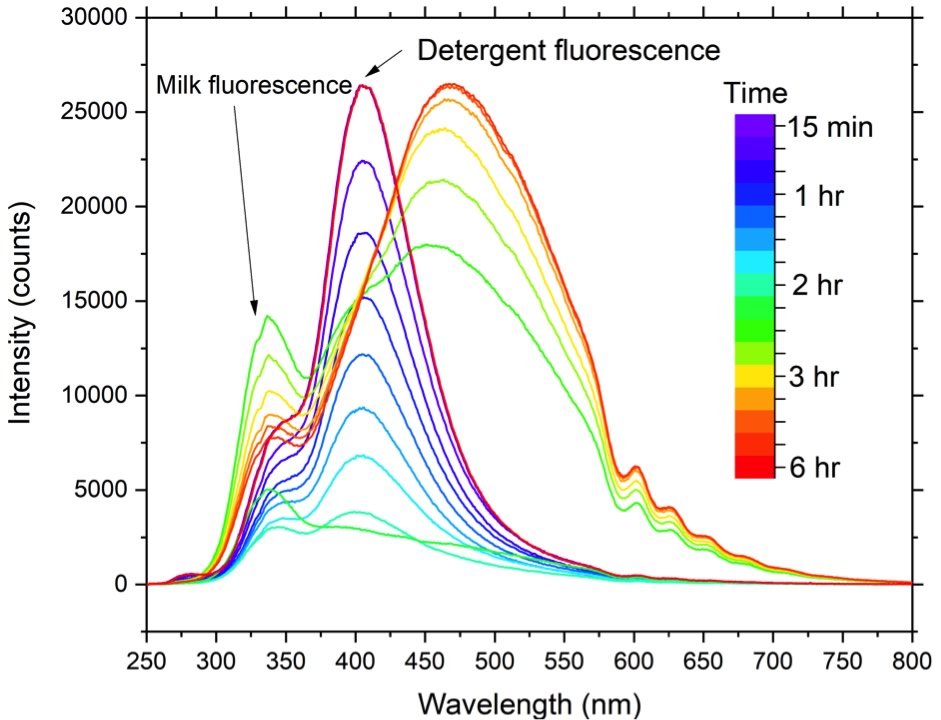
In-line monitoring of blend uniformity
Ingredient distribution within a blend or final product is another CPP. It is a requirement within the pharmaceutical and biopharmaceutical sector to perform an in process testing of blends to demonstrate adequacy of mixing however, the uniformity is not always assessed. With Raman spectroscopy, it is possible to probe the blend in- line or at-line to determine the uniformity and ingredient distribution, control, improve blending time, and prevent over blending, which may introduce aggregates that will affect the final quality of the product. This can be critical for pharmaceutical capsules/tablets since aggregates can lead to increased drug content above the recommended therapeutic dose for high potent drugs, for example Linoxin™, Xanax™ and Rocaltrol™.

Online monitoring of rinse samples for cleaning verification
Cleaning verification is one of the critical processes in all sectors of manufacturing but is heavily regulated in the biopharmaceutical and pharmaceutical sector. To prove the effectiveness and consistency of cleaning several checks are required. These are laborious checks and lead to production downtime. Implementation of a PAT system would provide rapid analysis and feedback on contaminant levels. Many ingredients have a response in the UV/vis – either direct absorbance or excitation and emission at a longer wavelength – and as a result, can be monitored using a PAT system. By applying UV/vis is it possible to monitor each step within a particular cleaning process which allows for the immediate release of the equipment at the end of the cleaning. The use of fluorescence spectroscopy is a novel application to cleaning verification and can determine the cleanliness of a surface and remove the ambiguity of visual inspection typically performed as part of the cleaning validation protocol.
Development of process automation to remove manual inspection
Manual inspection covers pre-production, in-line, and final product and can lead to unreliability, imprecision and high labour cost. The development of an automated inspection system would mitigate many of these problems and allow for high throughput of product and implementation of process monitoring tools to enable feedback to the manufacturing process. The following are the general categories of automated process inspection: 1) machine vision cameras or precision laser measurement sensors 2) turnkey automated inspection and gauging systems 3) combination of robots and cameras or precision laser for multiple inspections on more complex parts. The development of these systems can be a bespoke solution which requires a lot of expertise to develop. CAPPA has the capability to combine both spatial and chemical information into one inspection system to enable better performance over conventional inspection system. This technology is applicable in many sectors, such as food packaging, food quality control, medical device manufacturing and polymer coating.
Ingredient tracking in production processes
Certain ingredients have a higher value within the process chain. One example of this is protein within the production chain for baby powder. The protein is extracted from a raw ingredient, i.e. milk and thus is susceptible to natural variations due to seasonal factors. This can then affect the amount of protein that can be produced. To create the final product, several other ingredients are added throughout the process and can influence the protein concentration. A PAT system could be implemented that is capable to track protein levels at several different steps of the process and in various states, i.e. liquid or powder. Ingredient monitoring is mainly applicable to food and dairy sector since they encounter the most variability on the raw ingredients; however, there is scope to implement this in other sectors, such as polymer manufacturing (Monitoring Polymerization Reactions, raw material variability) and the pharmaceutical sector.


